
HorStem is the first registered veterinary medicine based on mesenchymal stem cells obtained from the umbilical cord of horses. Stem cells reduce lameness in horses with mild to moderate degenerative joint disease (OA).
It is a ready-to-use, sterile, injectable suspension for intra-articular use in horses. Each vial contains 15 million active stem cells, more than enough to achieve the minimum effective dose in the joint regardless of the degree of inflammation or the patient's immune system status.
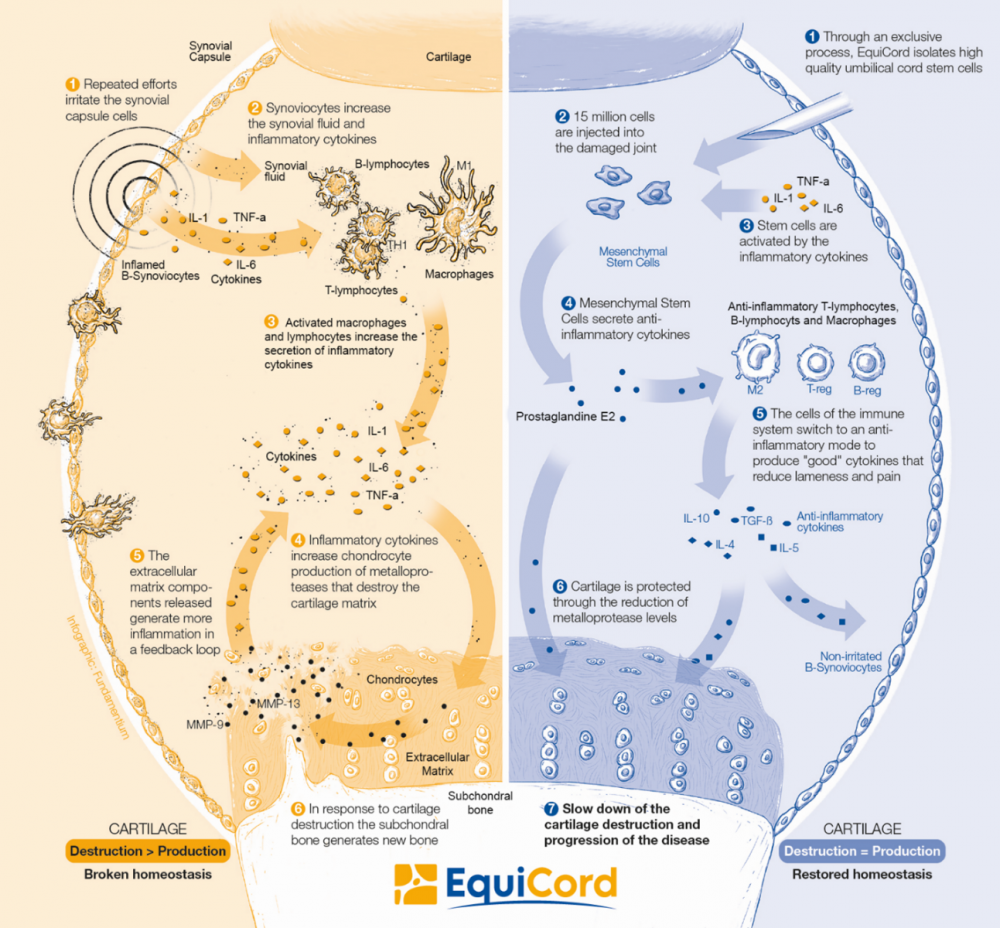
How does HorStem work?
The mechanism of action of HorStem is illustrated in the figure on the right. Mesenchymal stem cells are activated in the synovium by inflammatory cytokines. In response, they secrete anti-inflammatory cytokines such as Prostaglandin E2 (PGE2).
While PGE2 is primarily known for its pro-inflammatory effects, it also plays a crucial role in regulating inflammation. It is one of the major immunomodulatory cytokines in the body.
When applied to horses with OA, HorStem inhibits T-cell proliferation, reduces the production of pro-inflammatory cytokines like TNF-alpha, and decreases the presence of matrix metalloproteinases (MMPs) that contribute to the breakdown of cartilage's extracellular matrix. Studies have shown that these effects are mediated by PGE2. (1)
Moreover, umbilical cord-derived mesenchymal stem cells stimulate the production of anti-inflammatory cytokines such as IL-4, IL-5, IL-10, and TGF-beta. As the inflammatory response ceases and MMP levels drop, cartilage breakdown and disease progression halt.
Thus, stem cells create an ideal environment for tissue regeneration, leading to a decrease in inflammation and associated lameness in horses.

What are the results after treatment with stem cell therapy?
The effectiveness and safety of HorStem have been demonstrated in seven clinical studies conducted during its development. These studies have shown excellent results in treating horses with mild to moderate OA.(1)
The group treated with HorStem showed a greater reduction in lameness and pain scores compared to the placebo group. Furthermore, 75% of horses treated with HorStem had a lameness score reduced to 1 or lower (indicating no lameness or inconsistent lameness) by day 63. Clinical improvement persisted for over a year in 84% of treated horses.(1)
Does HorStem have side effects?
While the treatment is generally well-tolerated, it's important to be aware of potential side effects and how to minimize these risks.
Common side effects and preventive measures
A phenomenon known as "joint flare" is often reported in equine medicine following intra-articular administration of cellular products and conventional drugs.
Similarly, after treatment with HorStem, acute inflammation may occur in about 10% of horses within 24 hours, resulting in localized pain and significant lameness (flare). Significant improvement is expected within the next 48 hours, with complete resolution typically occurring within two weeks. Symptomatic treatment with non-steroidal anti-inflammatory drugs (NSAIDs) is a recommended option for these horses.
To reduce the risk of joint flares and mitigate their consequences, the following measures can be taken:
- Inform the owner about the risk of joint flare occurrence.
- Administer an NSAID alongside HorStem.
- In horses with high inflammation levels in the synovium, consider NSAID treatment two days before, on the day of, and for three days after the application of HorStem.
- Ensure HorStem is administered well in advance of any competitions to prevent potential inflammatory reactions from affecting planned events.
- For horses that have previously experienced joint flares, warn the owner of the risk of recurrence and follow the preventive plan outlined above.


Aftercare when using HorStem
Educate owners about potential side effects and instruct them to report any abnormalities immediately, so that any issues can be addressed in a timely manner. Additionally, schedule a follow-up visit to assess the response to treatment.
(1) Pradera Muñoz, A. Efficacy and safety study of allogeneic equine umbilical cord derived mesenchymal stem cells (EUC-MSCs) for the treatment of clinical symptomatology associated with mild to moderate degenerative joint disease (osteoarthritis) in horses under field conditions. 2019






